Accenture creates a regulatory document authoring solution using AWS generative AI services
AWS Machine Learning
FEBRUARY 6, 2024
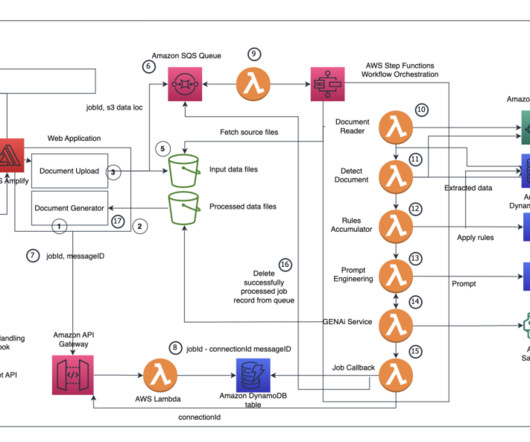
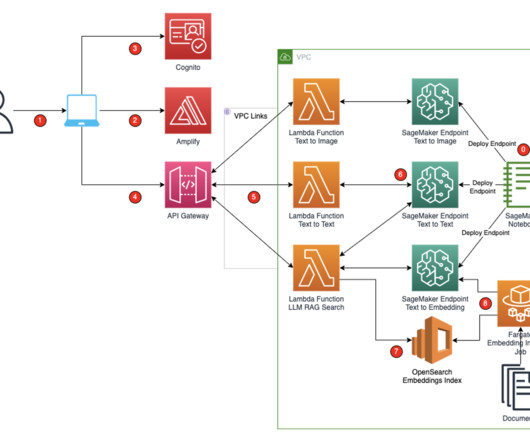
A key part of the submission process is authoring regulatory documents like the Common Technical Document (CTD), a comprehensive standard formatted document for submitting applications, amendments, supplements, and reports to the FDA. The tedious process of compiling hundreds of documents is also prone to errors.









Let's personalize your content